4-Methyltetrahydropyran MTHP
Features
High Polarity
- MTHP can be used in reactions requiring polar solvents, such as organometallic reactions.
Excellent Phase Separation with Water
- MTHP allows for the entire reaction and post-processing steps to be carried out with a single solvent.
- High extraction yield.
- MTHP reduces organics in wastewater.
High Stability Against Acids, Bases, and Oxidation
- Low risk of by-product formation, contributing to the acquisition of high-purity target compounds.
Others
- Depending on the reaction and substrate, it can achieve higher reaction yields compared to other ether-based solvent systems.
- MTHP allows extremely low-temperature reactions to be carried out at milder temperatures.
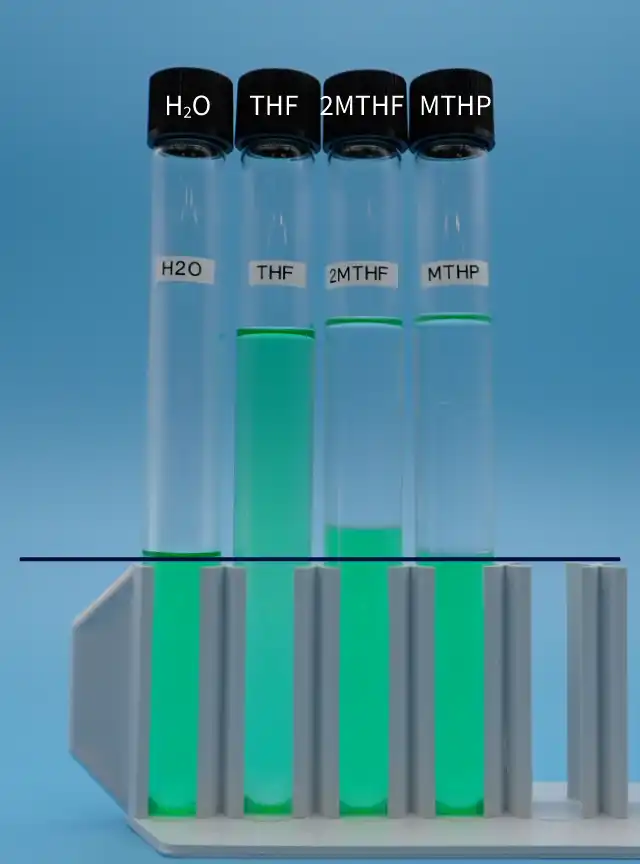
- Add 15 ml of water (colored green) to each test tube.
- For the three test tubes on the right, add 15 ml of THF, 2MTHF, and MTHP (not colored) to each respective test tube.
- Shake them at room temperature for 20 seconds, then let stand for 3 hours.
*2MTHF : 2-methyltetrahydrafuran
Applications
Reaction Solvent
- Organometallic reactions (e.g., Grignard reaction)
- Transition metal-catalyzed reactions (e.g., Suzuki-Miyaura coupling)
- Alternatives to aromatic and halogenated solvents
- Others (e.g., Azeotropic dehydration, Peptide synthesis)
Extraction Solvent
Polymerization Solvent
Coating

Physical Properties
| BP (℃) | MP (℃) | Specific gravity (20℃,g/cm3) | Viscosity(cP) | Flash point(℃) | Solubility in water(wt%) | Water solubility | Azeotropic point(℃)(H2O ratio) | SP value*(cal/cm3)^0.5 | |
|---|---|---|---|---|---|---|---|---|---|
MTHP | 105 | -92 | 0.86 | 0.78 | 6.5 | 1.5 | 1.4 | 85(19wt%) | 9.0 |
| THF | 65 | -109 | 0.89 | 0.55 | -15 | ∞ | ∞ | 64(6.0wt%) | 9.5 |
| 2MTHF | 80 | -136 | 0.85 | 0.60 | -11 | 14 | 4.4 | 71(11wt%) | 8.9 |
| CPME | 106 | -140 | 0.86 | 0.55 | -1 | 1.1 | 0.3 | 83(16wt%) | 8.4 |
| Et2O | 34.6 | -116 | 0.70 | 0.24 | -45 | 6.5 | 1.2 | 34(1.3wt%) | 7.6 |
| 1,4-Dioxane | 101 | 12 | 1.04 | 1.30 | 11 | ∞ | ∞ | 88(18wt%) | 10.0 |
/
*Calculated according to “Hansen solubility parameters a user’s handbook 2nd edition, CRC Press, ISBN: 0-8493-7248-8”
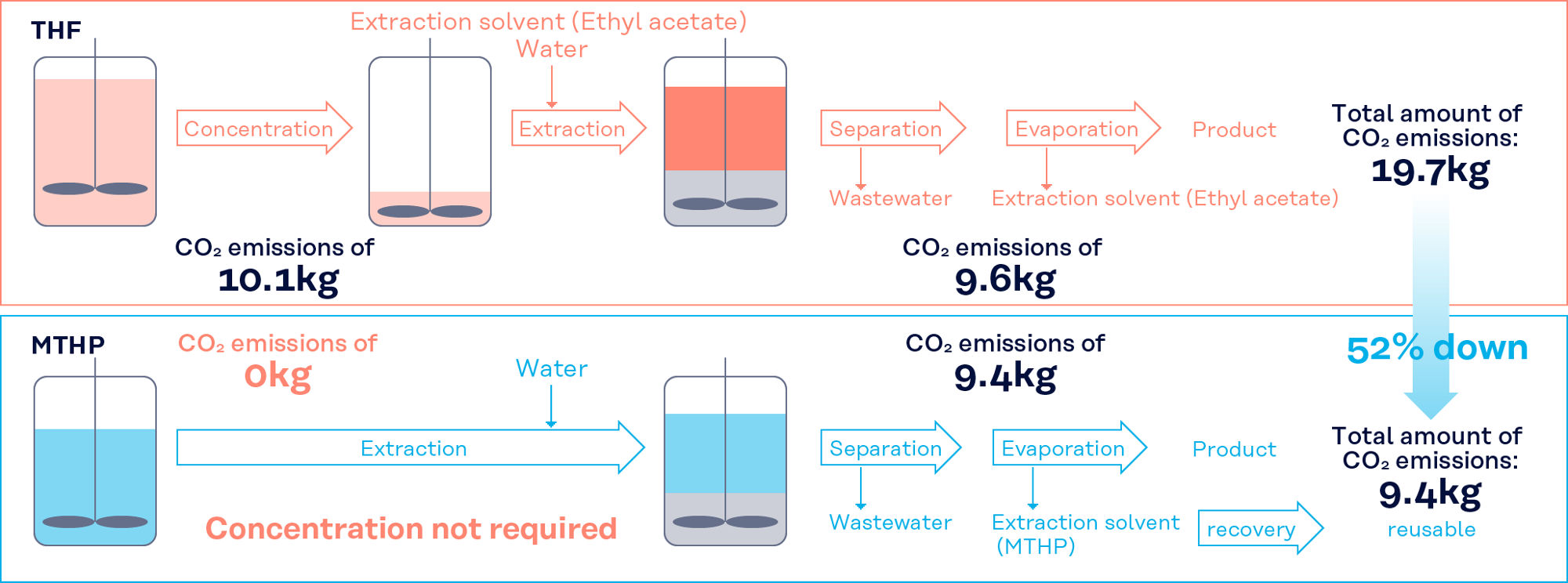
Process improvement
MTHP can be recovered and reused due to its good separation from water. In addition, the reaction solution does not need to be concentrated or extracted during post-processing, which saves energy and reduces CO2 emissions.
Example of Process Simplification and CO2 Reduction